KATHRYN JENKINS
When performing in-house haematology, or requesting a CBC from a reference lab, preparing a fresh blood film is important to help correctly identify an inflammatory response, and provides valuable prognostic information.
A recent study demonstrated the startling artefactual changes that occur to neutrophil morphology with prolonged sample storage. The appearance of cytoplasmic Döhle bodies can result in a false classification of toxic change in blood films made from stored blood, within as little as four hours after sampling1. This can lead to an erroneous assumption of the presence of a significant inflammatory response in the patient.
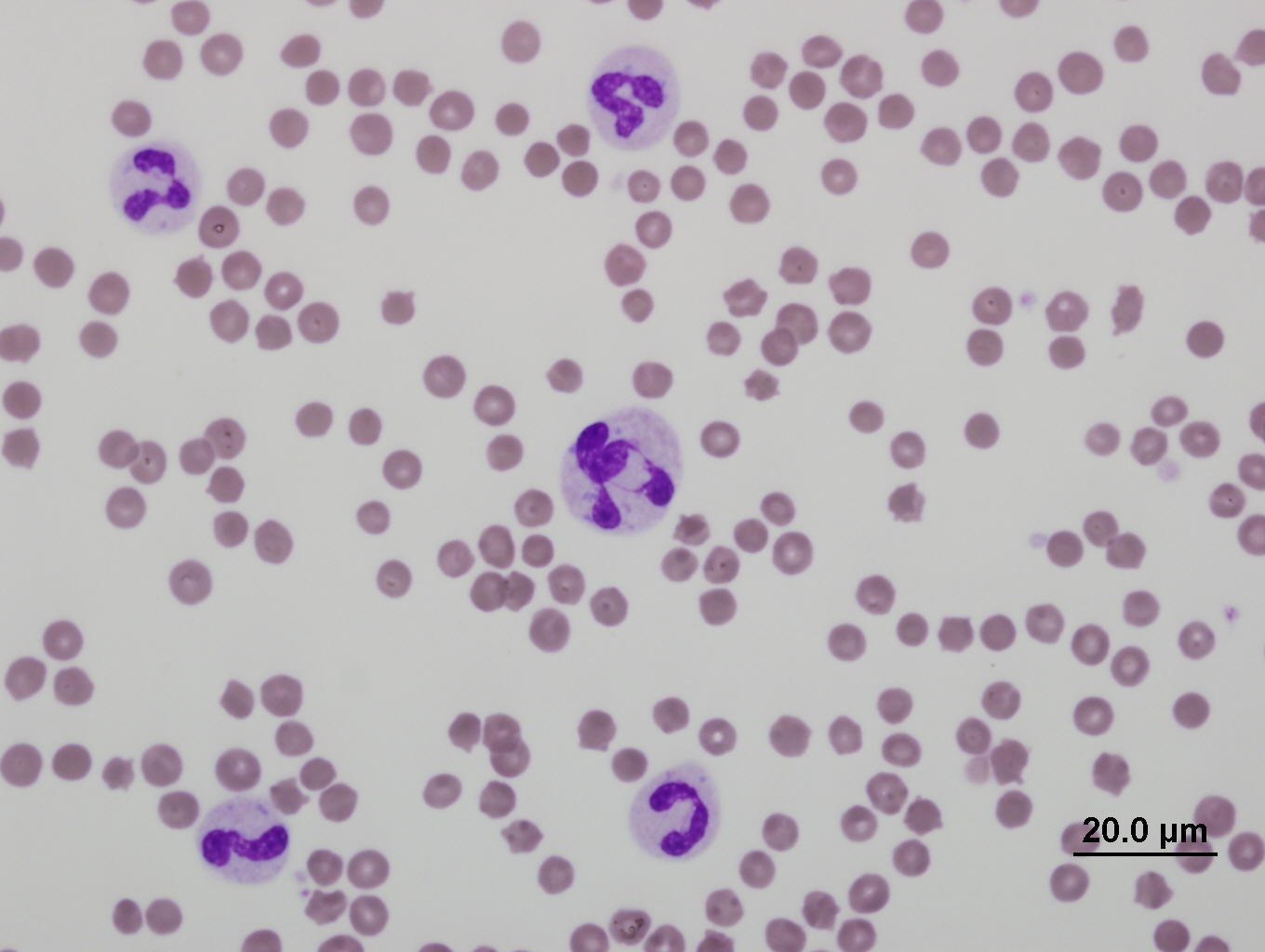
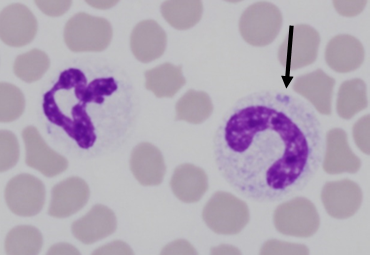
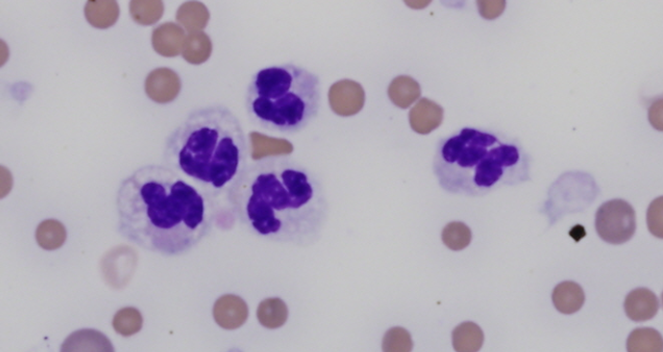
In companion animals, a classic inflammatory leukogram comprises neutrophilia with a left shift. The left shift refers to the presence of increased numbers of immature neutrophils in peripheral circulation, which are most often bands. Band neutrophils are characterised by a horseshoe-shaped nucleus, with smooth borders (Figure 1). The presence of even earlier precursor neutrophils (metamyelocytes or myelocytes) indicates more severe inflammation.
A left shift is often accompanied by so called ‘toxic change’. These are cytoplasmic morphologic features seen in neutrophils as a result of accelerated maturation through the bone marrow, in response to inflammatory cytokines. The presence of true toxic change in neutrophils has been associated with a poorer prognosis in dogs, cats and horses, including longer hospitalisation, increased treatment costs, and higher risk of mortality2,3,4.
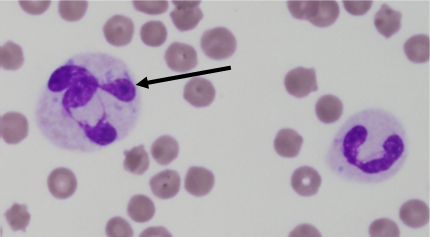
Toxic changes are considered biomarkers of inflammation. Most commonly, these include increased Döhle bodies (small light blue-grey, oval to amorphous intracytoplasmic inclusions of endoplasmic reticulum), cytoplasmic basophila, and foamy cytoplasmic vacuolation (Figure 1). Giant neutrophils and ring-shaped (donut) nuclei, are more commonly seen in cats and horses (Figure 2). The least common toxic change, is toxic granulation (retained primary granules), which indicates severe inflammation.
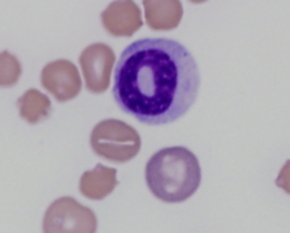
Storage changes in neutrophils that can mimic both toxic change and a left shift, include small Döhle-like bodies, crisp cytoplasmic vacuolation, and nuclear swelling (Figure 3). Storage change also affects other cell lines including increased haematocrit, increased MCV*, deccreased MCHC*, and echinocytosis in erythrocytes. Platelets can also appear swollen and pale, increasing MPV* and making them harder to identify on film reviews. Storage changes can be avoided by making a fresh blood film at the time of sampling.
Making a blood film gets easier with practice. Refer to our webinar on ‘The secret life of blood smears’ or see the Cornell University short instructional video on how to make a blood smear for some more great tips5.
*MCV: mean corpuscular volume / MCHC: mean corpuscular haemoglobin concentration / MPV: mean platelet volume
References
1. Effect of time and storage on toxic or pseudo‐toxic change in canine neutrophils. Bau-Gaudreault L, Grimes C. Vet Clin Pathol. 48:400-405, 2019.
2. Toxic Neutrophils in Cats: Clinical and Clinicopathologic Features, and Disease Prevalence and Outcome—A Retrospective Case Control Study. Segev G, Klement E, Aroch I. J Vet Int Med. 20:20-31, 2006.
3. Clinical, Biochemical, and Hematological Characteristics, Disease Prevalence, and Prognosis of Dogs Presenting with Neutrophil Cytoplasmic Toxicity. Aroch I, Klement E, Segev G. J Vet Int Med. 19: 64-73, 2005.
4. Association of Presence of Band Cells and Toxic Neutrophils with Systemic Inflammatory Response Syndrome and Outcome in Horses with Acute Disease. Lambert JL, Fernandez NJ, Roy M. J Vet Int Med. 30:1284-1292, 2016.