BERNIE VAATSTRA
… the three P’s of broad-spectrum investigation.
Calf diarrhoea is a frustrating and economically important disease responsible for more than half of all calf losses to weaning. Outbreaks are often multifactorial, involving interactions between infectious agents, calf and dam nutrition, environment, husbandry, and calf immunity. Successful treatment and prevention therefore require knowledge of the status of as many of these factors as possible.
Investigating calf diarrhoea outbreaks requires a broad-spectrum approach beginning with a thorough history taking and clinical exam. Age and origin of the calves, herd vaccination status, colostrum management, environment, physical condition and hydration status are important parts of the puzzle. In severe outbreaks where calves are dying or moribund, post mortem examination of one or more freshly dead or euthanased calves is extremely valuable.
In addition to the on farm investigation, a range of laboratory testing options help to further define the problem. It is tempting simply to hone in on faecal pathogen detection; while this is a key diagnostic aid, there are other tests that contribute to successful management and prevention of outbreaks. Diagnostic tests can therefore be categorised according to the three P’s: pathogen testing, evaluation of predisposing factors, and characterization of pathophysiologic changes.
Pathogen testing
Laboratory techniques used to target specific calf scour pathogens include faecal antigen immunoassay, culture, PCR, parasitology, and histopathology. These tests may be applied to faecal samples taken from sick calves or colonic contents retrieved from post mortem calves. It is important to note that detection of an organism does not necessarily prove causation. Results of pathogen testing always needs to be interpreted in light of the clinical signs, and preferably alongside appropriate gross and histopathological changes.
Faecal immunoassay
Rotavirus and cryptosporidium are the most frequently detected calf scour pathogens in calves up to around 5-weeks-old. Rapid faecal antigen ELISA testing provides convenient, sensitive and specific detection of these agents. Antigen ELISA testing is also available for enterotoxigenic E. coli expressing K99 antigen, usually diagnosed in young calves <1-week-old, and coronavirus, an infrequent cause of scours up to about 5-weeks-old.
Microbiology
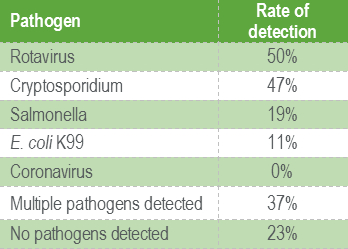
Salmonella enterica Typhimurium, and occasionally other serovars, may cause severe mucoid or haemorrhagic diarrhoea in calves of all ages. Diagnosis can be confirmed through culture in conjunction with appropriate clinical signs and pathology. Culture readily detects non-K99 strains of E. coli in normal and diarrhoeic calves, but usefulness is limited where strain typing is not available. Histopathology can be helpful for attaching and effacing strains. In older calves around weaning, Yersinia pseudotuberculosis is a consideration and can also be detected using culture.
Table 1 shows the percentages of different diarrhoea pathogens detected using faecal immunoassay and culture in calves less than 1-week-old. This panel uses faecal antigen testing to detect Rotavirus, Cryptosporidium, E. coli K99 and Coronavirus, while Salmonella is detected using culture.
Parasitology
Calves approximately 4-weeks-old onward are susceptible to coccidiosis. Clinical signs of diarrhoea +/- blood are related to maturation of oocysts in the large intestine during days 18-19 of infection. Faecal oocysts are present in highest numbers during days 22-25 of infection, therefore detection of only small numbers of oocysts does not rule out significant disease. Oocysts are readily detected using traditional parasitology methods. As calves approach weaning and increase the percentage of pasture in the diet, nematode faecal egg counts provide a rough guide to the level of parasite challenge and may help inform parasite management.
PCR
Most of the calf diarrhoea pathogens are detected rapidly and reliably using immunoassays, microbiology, or parasitology. Exceptions include attaching and effacing E.coli strains, BVD virus, and bovine adenovirus. PCR may be useful for detection of viral pathogens in certain circumstances.
BVD may have a role in neonatal scours through immunosuppression leading to increased susceptibility to other pathogens, or through transient or persistent infection. BVD PCR is recommended for testing calves <35 days old, while antigen ELISA can be used for older calves. ELISA testing can be done on serum, PCR on serum and skin.
Bovine adenovirus type 10 is a differential for diarrhoea and deaths in slightly older calves, particularly around the time of weaning and afterwards. A qPCR assay is available for use on EDTA blood, fresh, or fixed tissue.
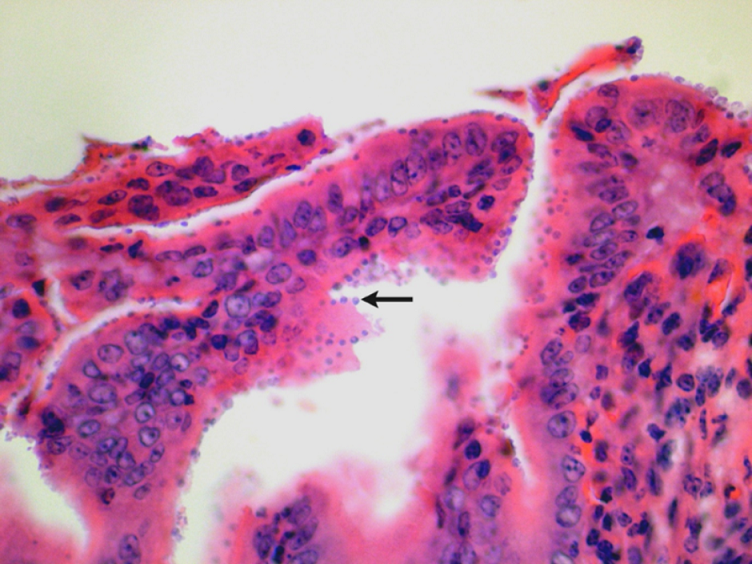
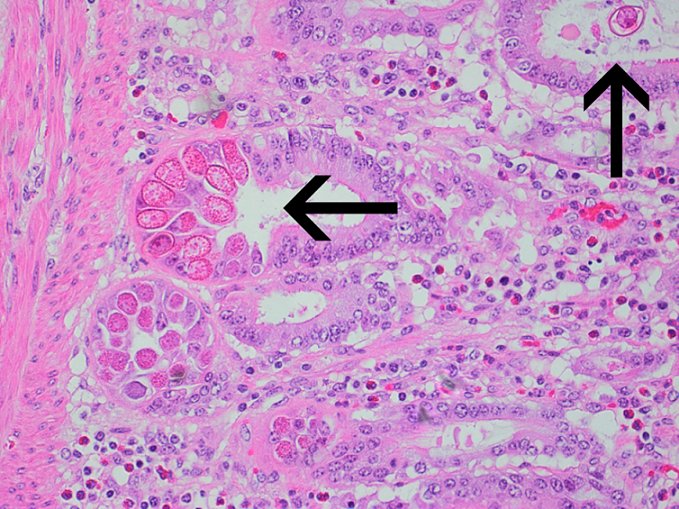
Histopathology
Histopathology can be of great value in calf scour outbreaks. Firstly, it may help to confirm the significance of any pathogens detected by demonstrating typical pathological features (e.g. intestinal villus atrophy with rotavirus and coronavirus, pseudomembranous enteritis with salmonellosis). Secondly, organisms or their footprints may be identified. Examples include E. coli lining up on enterocytes, Yersinia spp. colonies, cryptosporidia populating apical enterocytes (Figure 1), coccidial stages within crypts of the distal small intestine and large intestine (Figure 2), and adenoviral inclusion bodies (Figure 3). Thirdly, pathogens or conditions not detected using the usual methods may be suspected or detected using histopathology (attaching and effacing E. coli, rumen acidosis, adenovirus, and peritonitis).
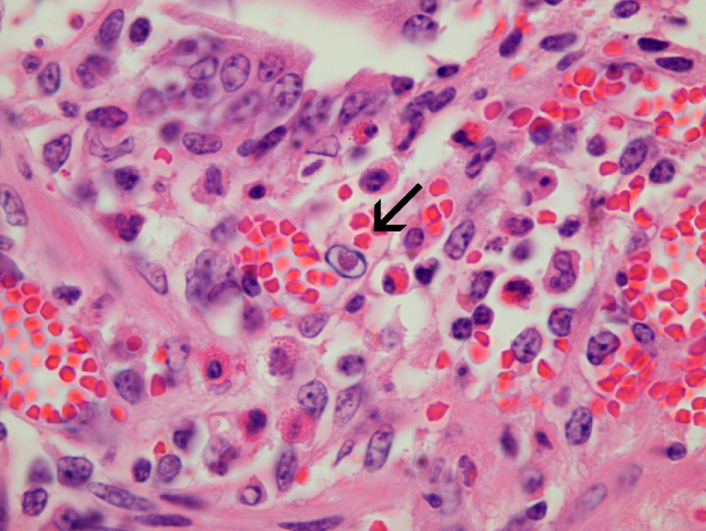
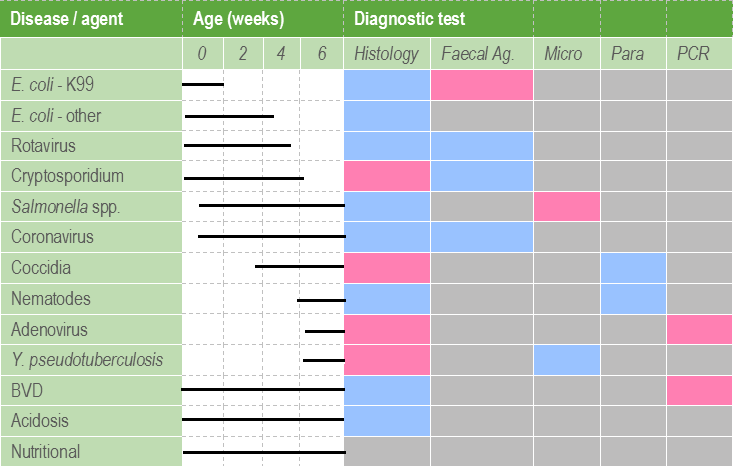
It is important to note that the value of histopathology may be substantially limited by tissue degradation as a result of autolysis. The delicate GI mucosa starts to deteriorate within minutes after death and after as little as 30 minutes, changes such as villus atrophy may be missed. Organisms such as cryptosporidia and coliforms may also be lost from the mucosal surface. Therefore it is best to take histological samples from a very recently dead calf, or even better, euthanase a moribund calf. Multiple sections of rumen, omasum, abomasum, small and large intestines should be collected immediately, partially opened, and immersed in an appropriate volume of 10% formalin before proceeding to the rest of the post mortem. Preservation of the mucosa may be aided by injecting formalin into the lumen of the bowel sections collected. Table 2 demonstrates the utility of the different diagnostic tests used in the investigation of calf scours.
Predisposing factors
In addition to looking for specific pathogens, some of the underlying factors contributing to diarrhoea may be investigated. BVD virus has already been mentioned and may be tested as outlined above.
Failure of passive transfer is an important contributor to calf diarrhoea. While the gold standard test for assessing passive transfer is IgG immunoassay, there are two other tests that can be used as rapid and cost-effective proxies. Serum total protein (TP) is convenient in that calves 0-8 days can be
tested without knowing the exact age of the calf. A cut-off of >53g/L is used as evidence of adequate transfer of colostral immunoglobulin (Cuttance et al. 2017). However, it is important to note that calves with diarrhoea are likely to be dehydrated and have proportionately elevated TP.
In situations where calves are sick and scouring, serum gamma glutamyl transferase (GGT) may be a helpful indicator of colostral transfer (Thompson & Pauli, 1981). The age of the calf is required in order to interpret the results. GGT levels indicate that sufficient colostrum was ingested, but do not give any information about the quality of the colostrum. Therefore GGT needs to be interpreted in light of colostrum storage practices and colostrum quality (e.g. using Brix refractometer).
Milk powder testing may be considered if there is a suspicion that nutritional factors are contributing to scours. Curd testing assesses the casein content and quality, while fat, protein and lactose testing can indicate how much energy is supplied by the fat fraction.
Pathophysiology
As well as looking at aetiological and predisposing factors to calf diarrhoea, successful treatment of clinical cases also requires an understanding of the pathogenesis.
Routine biochemistry and haematology can help evaluate some of the physiological changes taking place. These include dehydration, manifested by clinical signs such as skin tenting and sunken eyes and in blood work as haemoconcentration and hyperproteinaemia (unless complicated by haemorrhage); Metabolic acidosis develops due to loss of bicarbonate into the GI tract, L-lactic acid production in poorly perfused tissues, and D-lactic acid production in the colon due to increased colonic fermentation. If unchecked, this process may lead to CNS depression, recumbency, and coma. Serum bicarbonate can be used in addition to clinical signs to estimate level of acidosis and base deficit in order to guide bicarbonate requirements. A VetScript article by Sandra Forsyth included a helpful table to estimate base deficit in calves in order to calculate bicarbonate requirements in fluid replacement therapy (Forsyth 2018).
Electrolyte imbalances go along with dehydration and need to be considered when calculating fluid replacement therapy. There may be a total body deficit of sodium, chloride, and potassium due to intestinal fluid loss and decreased intake. However, serum biochemistry will often show hyponatremia, normochloremia, and sometimes hyperkalemia. The latter occurs in metabolic acidosis, due to the exchange of extracellular hydrogen ions for intracellular potassium ions in order to buffer the ECF. Hyperkalemia results in cardiac arrhythmias. Other biochemical and haematology changes can include evidence of energy deficit (hypoglycaemia, ketosis) and inflammation (neutrophilia with left shift, hyperfibrinogenemia).
In conclusion, thorough work up of calf diarrhoea cases can be complicated and may require evaluation of many diagnostic parameters, clinical, and environmental factors. However, putting all the pieces together in order to diagnose, treat, and prevent scour outbreaks is highly satisfying and will improve animal and farmer welfare.
References
· Cuttance EL, Mason WA, Denholm KS, Laven RA. Comparison of diagnostic tests for determining the prevalence of failure of passive transfer in New Zealand dairy calves. New Zealand Veterinary Journal, 65:6-13, 2017.
· Forsyth S. Calf diarrhoea. VetScript, 31:56-8, 2018.
· Thompson JC, Pauli JV. Colostral transfer of gamma glutamyl transpeptidase in calves. New Zealand Veterinary Journal, 29:223-6, 1981.
This article was previously published in VetScript, October 2018.

